How To Find Entropy Of System
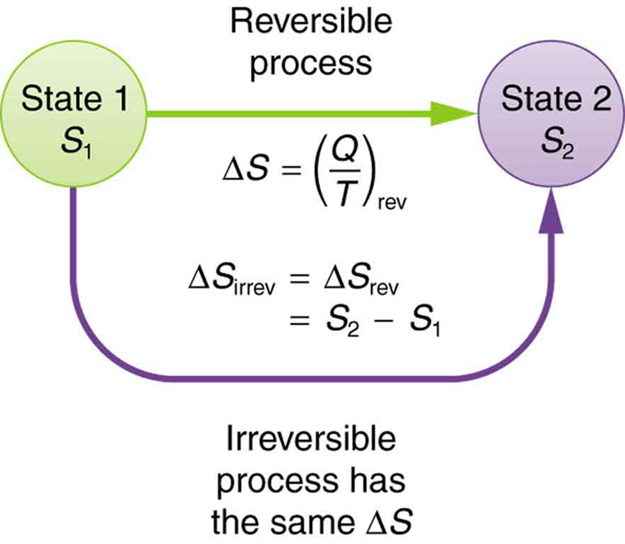
The entropy change of the reservoir is. The content of Equation is that the entropy of a system can be altered in two ways.
This question hasnt been solved yet Ask an expert Ask an expert Ask an expert done loading.
How to find entropy of system. The entropy change of the device is zero because we are considering a complete cycle return to initial state and entropy is a function of state. Moreover if the reaction of the process is known then we can find by using a table of standard entropy. We calculate isochoric entropychange by Reversible heatingcooling at constant P reversible isobaric.
In this case it is useful to remember that dq nC_pdT So dfracdqT nC_p dfracdTT Integration from the initial to final temperature is used to calculate the change in entropy. Show transcribed image text Expert Answer. We begin by using the first law of thermodynamics.
Question 1 How can we prove that entropy is property of a system. A decrease in disorder. TT TT Paragraph Aria DO ESTE Of Malupe 16 3712pt ET T TA OSS ws Pathp.
ΔS 0 2S 0 NH 3 - S 0 N 2 3S 0 H 2 ΔS 0 21925 - 1915 31306 ΔS 0 -1983 Jmol K. When a system cools its entropydecreases. S k lnW S k ln W.
It can also be calculated from the change in heat content in a system divided by the temperature. A microstate W is a specific configuration of the locations and energies of the atoms or molecules that comprise a system like the following. This capability was introduced in Linux version 1330.
Entropy is broadly defined as loss of the ability for a system to do work. Total starting entropy 186 2205 596 J K-1mol-1 You ended up with 1 mole of carbon dioxide and two moles of liquid water. Entropy as a Measure of the Multiplicity of a System The probability of finding a system in a given state depends upon the multiplicity of that state.
I through heat exchange and ii through irreversibilities. But Im not sure these answers will help you much in understanding what entropy is. The negative sign of enthalpy change -2045 kJ shows that the system has lost 2045 kJ of energy to surroundings.
The change in entropy is defined as. The lost work in Equation 64 is always greater than zero so the only way to decrease the entropy of a system is through heat transfer. A C2H8g 5 O2g 3 CO2g 4H2Og ΔH -2045 kJ the reaction takes place at 2 5oC This reaction is an exothermic reaction.
DE dQ - dW where E is the internal energy and W is the work done by the system. A C 2 H 8 g 5 O 2 g 3 CO 2 g 4H 2 Og ΔH -2045 kJ b H 2 Ol H 2 Og ΔH 44 kJ Solution The change in entropy of the surroundings after a chemical reaction at constant pressure and temperature can be expressed by the formula ΔS surr -ΔHT where. The Linux kernel generates entropy from keyboard timings mouse movements and IDE timings and makes the random character data available to other operating system processes through the special files devrandom and devurandom.
Besides there are many equations to calculate entropy. There are some Linux kernel patches allowing one to use more entropy sources. Calculate the entropy of the surroundings for the following two reactions.
If the happening process is at a constant temperature then entropy will be Derivation of Entropy Formula is the. It would appear that the process results in a decrease in entropy - ie. From the balanced equation we can write the equation for ΔS 0 the change in the standard molar entropy for the reaction.
Here a state is defined by some measurable property which would allow you to distinguish it from other states. For gases there are two possible ways to evaluate the change in entropy. The total entropy change is the sum of the change in the reservoir the system or device and the surroundings.
For changes in which the initial and final pressures are the same the most convenient pathway to use to calculate the entropy change is an isobaric pathway. Here Q is the heat transfer necessary to melt 100 kg of ice and is given by Q mLf where m is the mass and Lf is the latent heat of fusion. The entropy change is negative - with a decrease in temperature andpositive with an increase in temperature.
That is to say it is proportional to the number of ways you can produce that state. Following the work of Carnot and Clausius Ludwig Boltzmann developed a molecular-scale statistical model that related the entropy of a system to the number of microstates possible for the system. Entropy Example.
Total entropy at the end 214 2699 3538 J K-1mol-1 Entropy change what you end up with - what you started with. The entropy change of the surroundings can be calculated by the equation d S s u r d q T s u r regardless of the path irreversible or reversible. Lf 334 kJkg for water so that Q 100 kg 334 kJkg 334 10 5 J.
Calculate the entropy of the surroundings for the following reaction. Delta Sfrac Q T.

Entropy Intuition Video Thermodynamics Khan Academy

Calculate The Entropy Change In The Surroundings Youtube

How To Calculate Entropy Changes Mixing Ideal Gases Youtube

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

15 3 3 Calculate The Standard Entropy Change For A Reaction Hl Youtube

Entropy Balance Equation An Overview Sciencedirect Topics
Measuring Entropy And Entropy Changes Introductory Chemistry 1st Canadian Edition
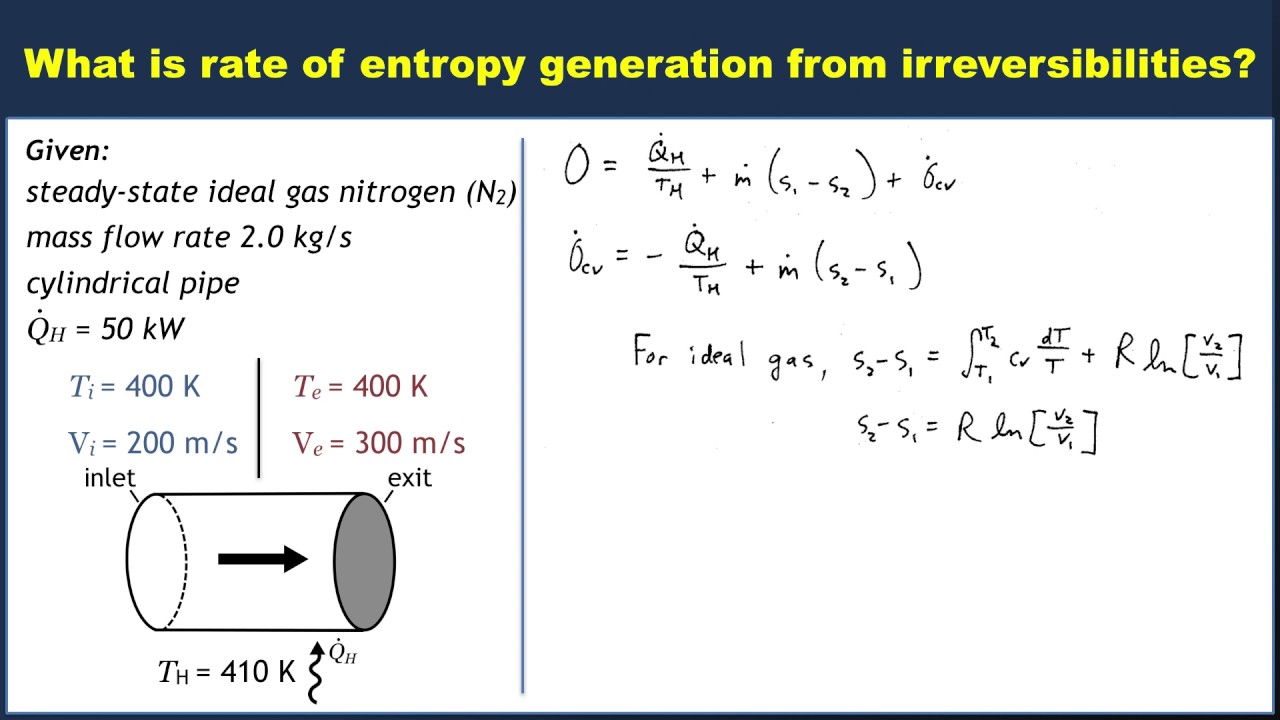
Example Entropy Balance In An Open System Youtube

Entropy Change For Melting Ice Heating Water Mixtures Carnot Cycle Of Heat Engines Physics Youtube

How To Calculate Entropy Changes Liquids Solids And Phase Changes Youtube

Calculation Of Entropy Change In Irreversible Cycles Meaning Of Delta Q T In Irreversible Processes Physics Stack Exchange

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

Changes In The Overall Entropy Of A Single Biological Organism During Lifetime
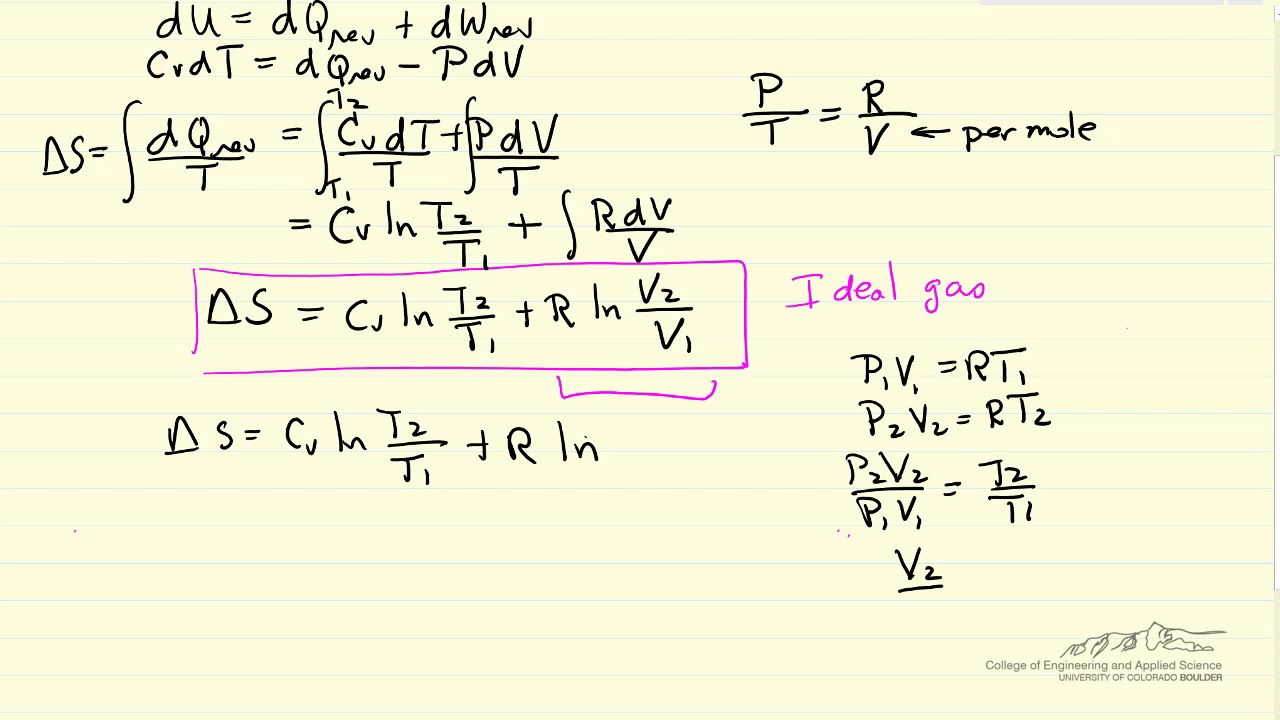
How To Calculate Entropy Changes Ideal Gases Youtube

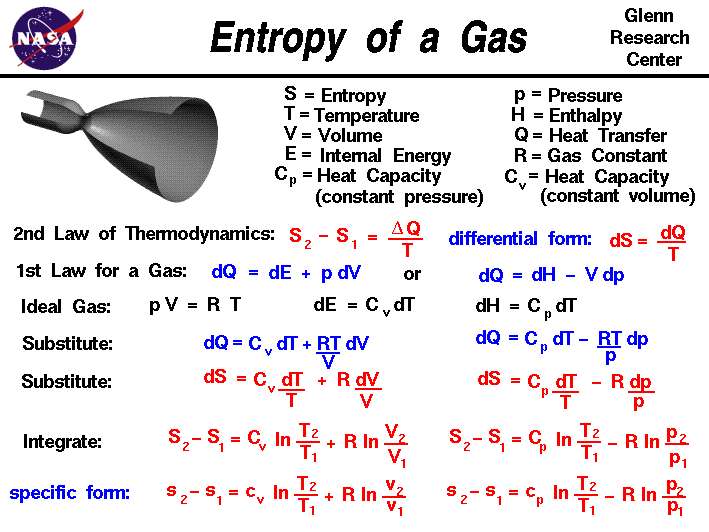

Post a Comment for "How To Find Entropy Of System"